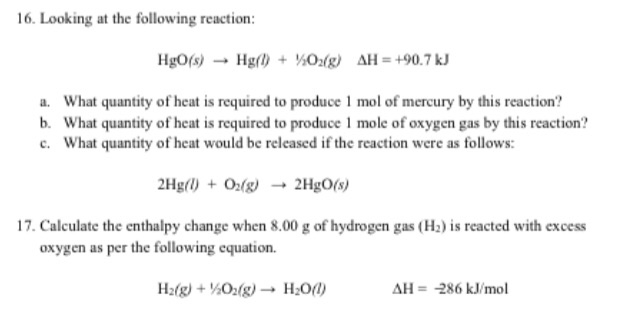
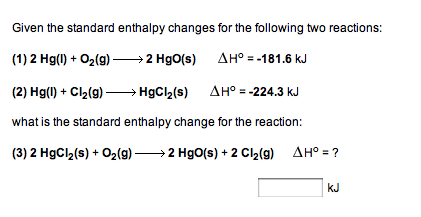
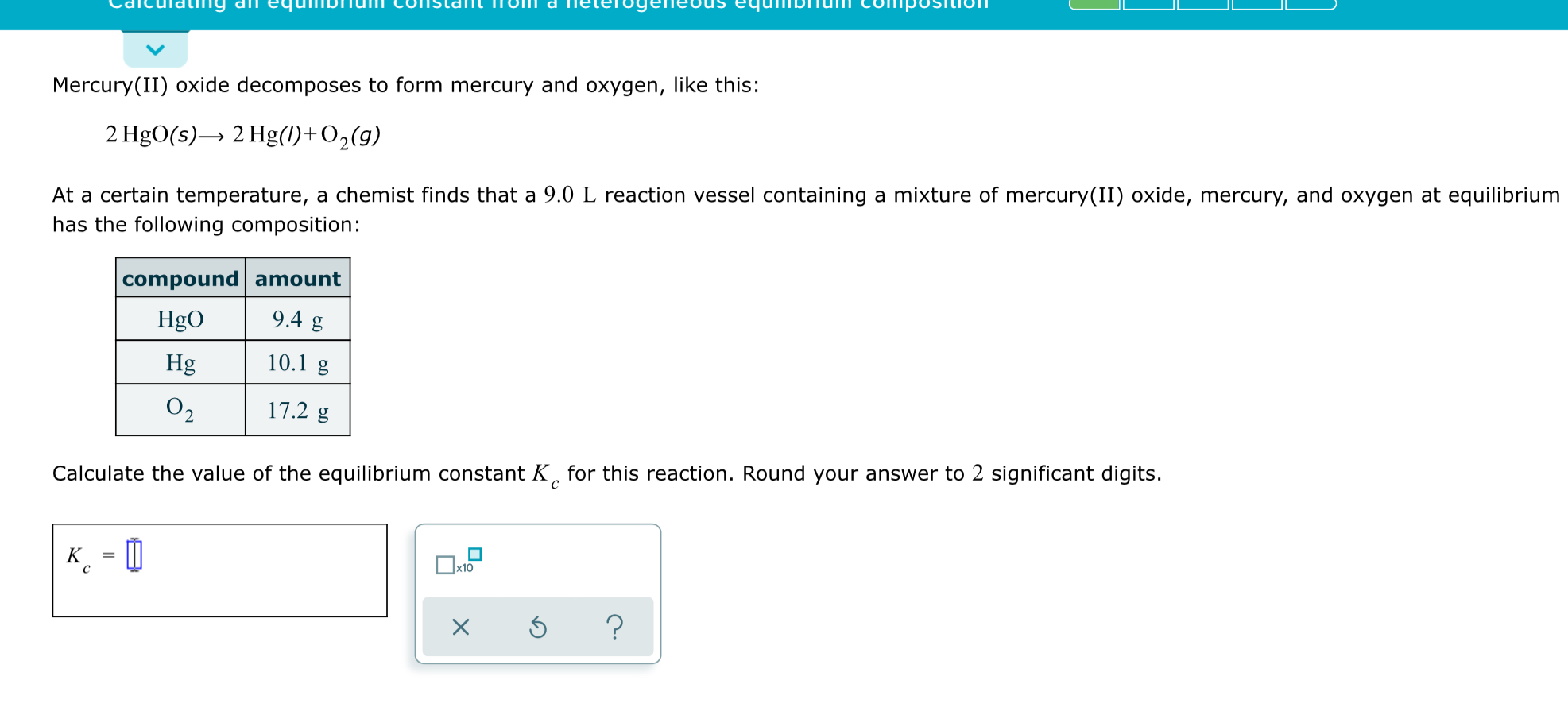
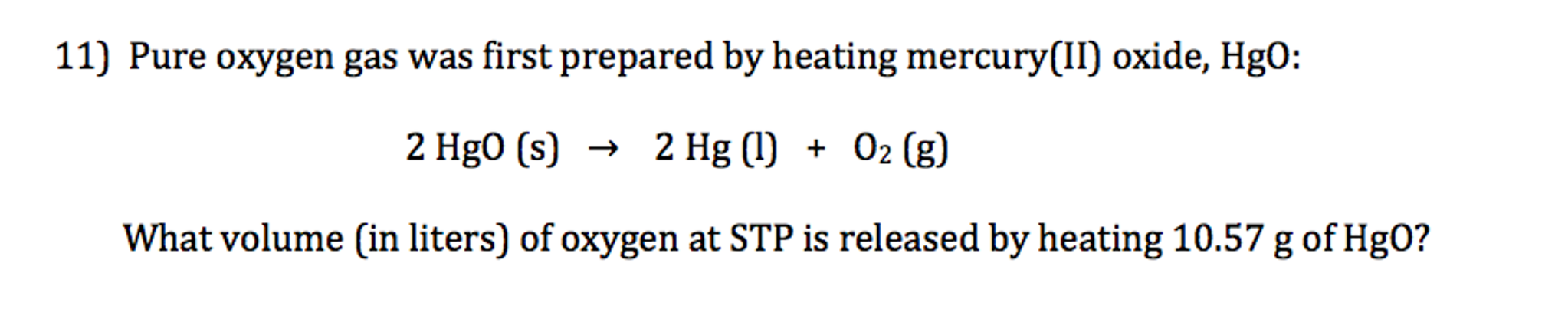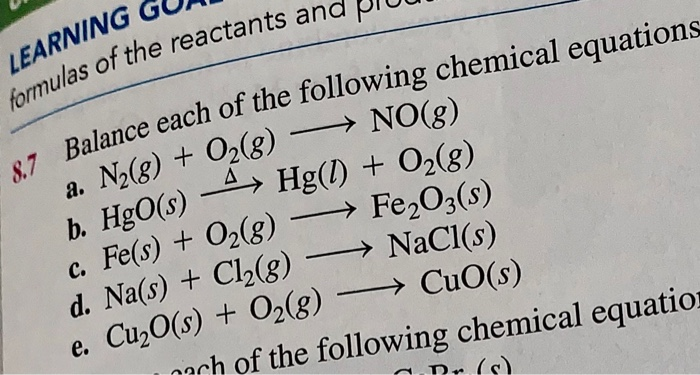Hgo S Hg L O2 G
I it is a reverse reaction of the original reaction hgo s hg l o2 g ii it is obtained by multiplying original reaction by 2.
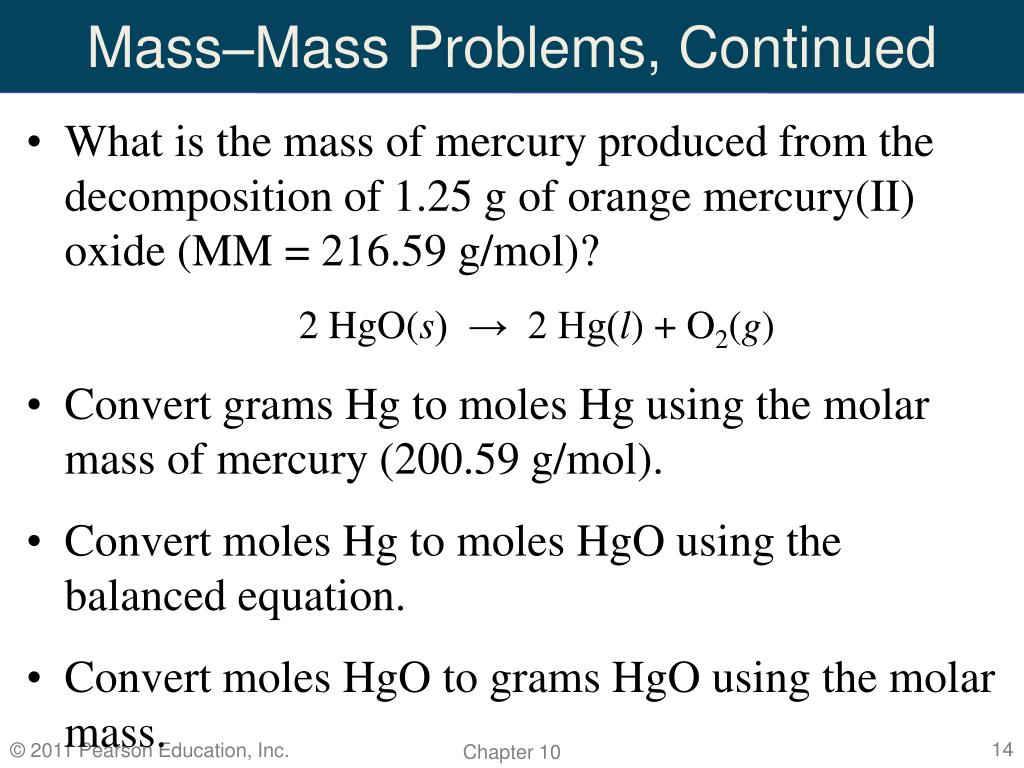
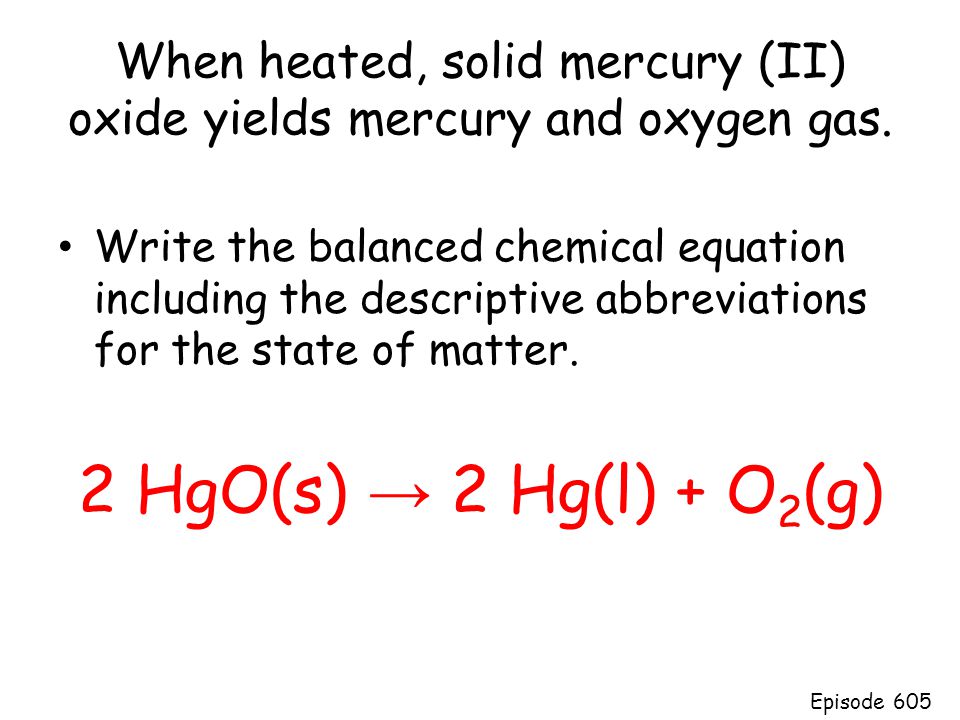
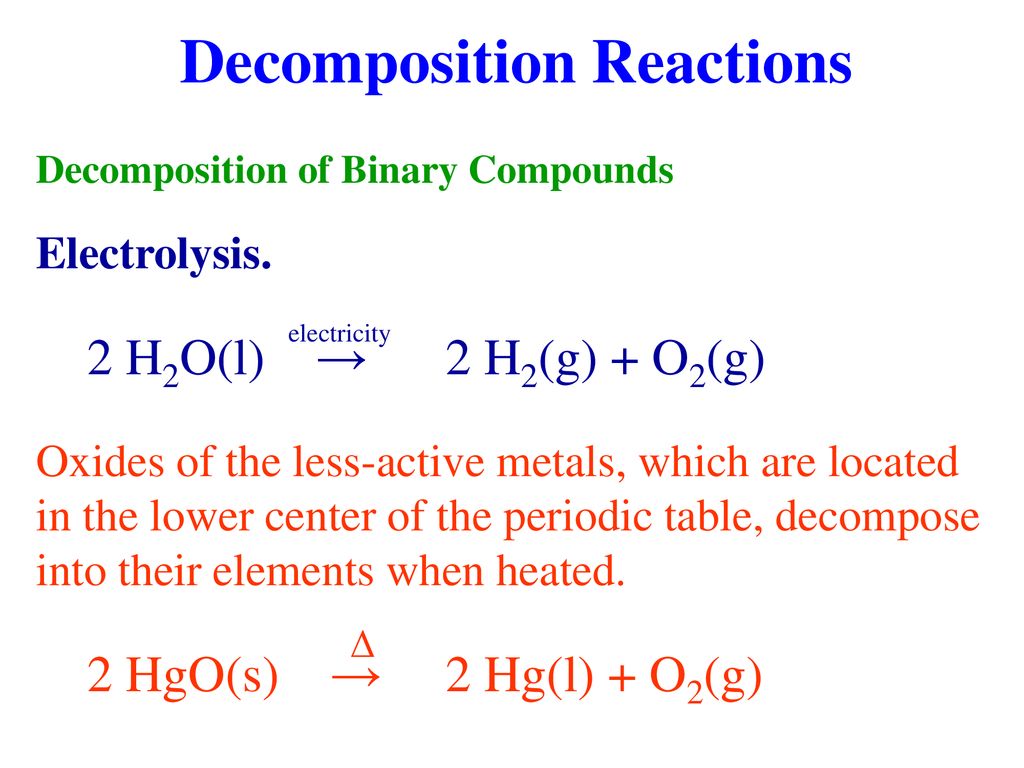
Hgo s hg l o2 g. How to balance equations. 2 h g o s h e a t 2 h g l o 2 g. If you do not know what products are enter reagents only and click balance. Mercuric oxide h g o decomposes on heating to give mercury h g and oxygen o 2.
What weight of elemental mercury will be obtained by the decomposition of 0 125 moles of hgo. Inclua sua resposta e ganhe pontos. You can use parenthesis or brackets. 2 hg l o2 g 2 hgo s the reaction given in part c is exothermic i e heat is released.
Now since hgo and hg is a 2 2 ratio which is a 1 1 ratio n hg will have the same mol as hgo. It is a decomposition reaction and the balanced equation can be written as. Correct ok new questions in science. For example c6h5c2h5 o2 c6h5oh co2 h2o will not be balanced but xc2h5 o2 xoh co2 h2o will.
In many cases a complete equation will be suggested. The valency of oxygen is 2 why what is colour of of sun. 2 hgo s 2 hg l o2 g reaction type. Una aplicación para balancear y completar ecuaciones químicas.
First you find the mol of hgo. N hgo mass molar mass 94 5 216 6 0 4375 mol. We can see that. Since you have the mol you can find out how much mass is needed for you elemental mercury.
Compound states like s aq or g are not required. What is the occupation done to get finished products from raw material. Using this eqn mass molar mass x mol. Balanceamento das equações químicas abaixo e depois indique qual o tipo de reação elas representam síntese análise simples troca ou dupla.
Ecuacion química completada y balanceada 2 hgo 2 hg o2 con productos calculados. N hgo n hg 0 4375 mol. Given the reaction 2 hgo s 2 hg l o2 g. Hgo s hg l o2 g 1 ver a resposta army5 está aguardando sua ajuda.
Snowtx snowtx oque e pra ser feito. Replace immutable groups in compounds to avoid ambiguity. Ccupation is called indusuy 1. Compound states like s aq or g are not required.